How to Calculate Specific Heat: A Practical Guide for 2025
Understanding specific heat and how to calculate it is essential for students and professionals alike in various fields, including physics, chemistry, and engineering. This guide will take you through the fundamental concepts, formulas, and practical applications of specific heat capacity, ensuring you possess a thorough knowledge of both the theory and practical methods for calculating specific heat as we approach 2025.
Understanding Specific Heat Capacity
The **specific heat capacity** is defined as the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius (°C). It plays a crucial role in various thermodynamic processes. The definition of specific heat can vary slightly when considering the various states of matter, as different substances possess unique properties that affect how heat is absorbed or released.
Definition and Units of Specific Heat
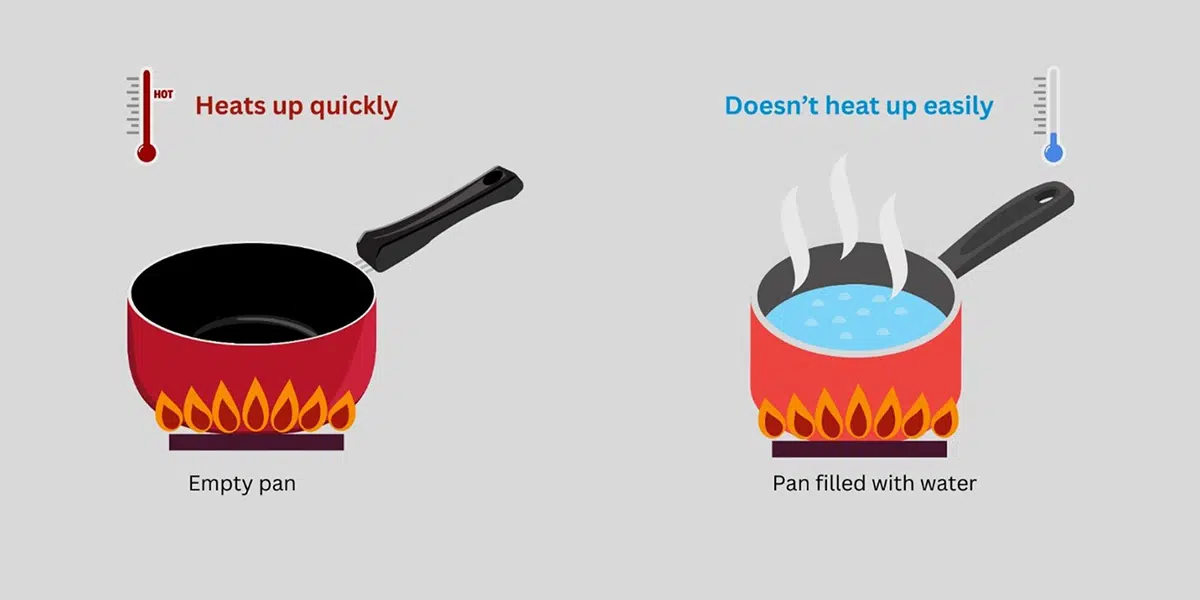
The specific heat definition helps us understand thermal energy transfer. The units of specific heat are typically expressed in joules per gram per degree Celsius (J/g°C) or calories per gram per degree Celsius (cal/g°C). For example, the **specific heat of water** is approximately 4.18 J/g°C, making it one of the substances with the highest specific heat capacities, vital for regulating temperatures in chemical and biological processes.
Formula for Specific Heat
The formula for specific heat can be derived from the relationship between heat transfer and temperature change. This can be expressed with the equation:
Q = mcΔT
Where:
- Q = heat absorbed or lost (in joules)
- m = mass of the substance (in grams)
- c = specific heat capacity (in J/g°C)
- ΔT = change in temperature (final temperature – initial temperature)
Using this formula, you can calculate how much energy is required to change the temperature of a substance, providing a practical approach to calculating specific heat during experiments and applications.
Factors Affecting Specific Heat
<pSeveral factors influence the specific heat of a material. The material’s state (solid, liquid, or gas) significantly impacts its capacity to store heat. For example, liquid water has a high specific heat of water, making it an excellent coolant. Similarly, the molecular structure and bonding of materials also contribute to their specific heat values—metals typically have lower specific heats compared to fluids due to their closely packed particle arrangement, which allows for more efficient thermal conduction.
Methods for Measuring Specific Heat
There are various specific heat measurement methods used across different applications, from laboratory settings to industrial scenarios. The choice of method often depends on the accuracy required and the type of substance being analyzed. Here we will explore some practical methods.
Calorimetry in the Laboratory
One of the most common methods of determining specific heat is calorimetry, which measures the heat transfer to or from a substance during a temperature change. In a typical calorimetry experiment, known masses of substances with a measured initial temperature are mixed. By observing the temperature change, researchers can use the specific heat formula to calculate the specific heat capacity. Students in the classroom often conduct specific heat experiments using simple materials such as water and metal to see these concepts in action, often leading to engaging learning experiences.
Using a Specific Heat Calculator
For those who prefer a quicker approach, a specific heat calculator can simplify the process of calculating specific heat. Many online tools are available where you can input values for heat transfer, mass, and temperature change, and the calculator will output the specific heat capacity for you. This method is especially helpful for rapid calculations in industrial applications, where timing is crucial.
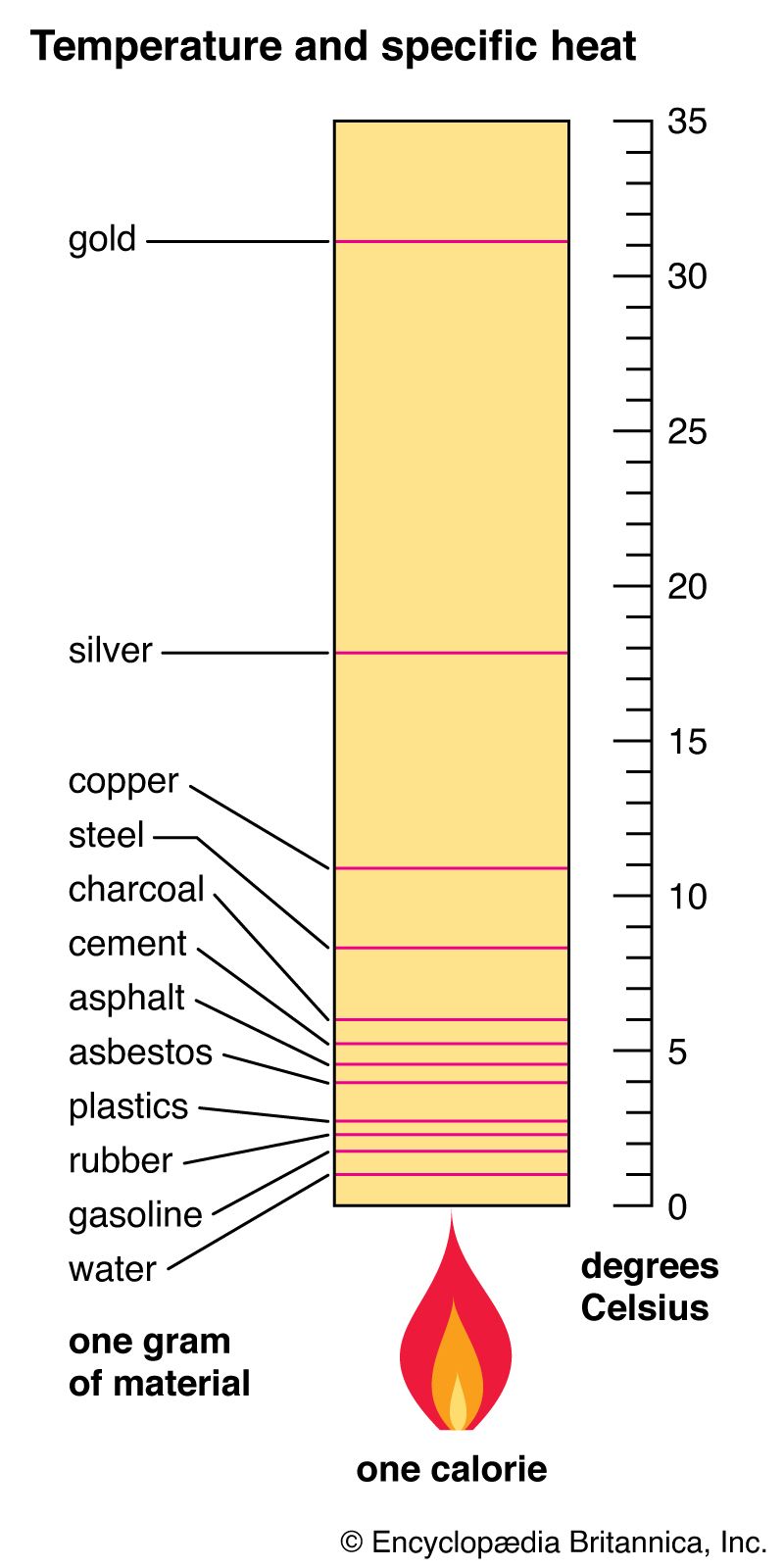
Comparative Analysis with Reference Tables
Another practical approach to understanding specific heat involves using a specific heat reference table that lists the specific heat values of various materials. Having quick access to this information can enhance decision-making processes in fields such as engineering or material science. Knowing the specific heat of metals compared to that of water can assist engineers in selecting appropriate materials for heat management in systems.
Applications of Specific Heat in Real Life
The complexity of the **specific heat value** extends into many aspects of everyday life and technology. From cooking to climate control, understanding how heat interacts with substances is vital for optimizing processes. Here we will discuss some practical applications.
Specific Heat in Engineering and Environmental Science
In engineering, specific heat plays a crucial role in designing thermal management systems, including HVAC (heating, ventilation, and air conditioning) systems. Understanding how materials react to temperature changes can help engineers design more energy-efficient systems. In environmental science, knowledge of specific heat enables researchers to study thermal changes in ocean currents or climate patterns, contributing valuable insights to studies on global warming and energy balance.
Specific Heat in Chemistry and Cooking
In chemistry, the concept of specific heat in thermodynamics is essential for understanding reaction energies and reactions themselves. Similarly, cooking recipes often consider the specific heat of ingredients to achieve perfectly cooked meals, such as understanding the **specific heat of liquids** versus solids. For example, adding ice to smoothies requires knowledge of the **specific heat of ice** to predict how it affects temperature variations during the blending process.
Specific Heat Applications in Daily Life
Understanding the implications of specific heat allows everyday consumers to make informed decisions. For example, when choosing cookware materials (like copper, stainless steel, or cast iron), a chef’s understanding of their different specific heats will guide their performance. The **specific heat of air** also plays a role in building and maintaining a comfortable home environment, where calculations around thermal insulation materials can significantly impact energy costs.
Key Takeaways
- The definition of specific heat is vital for processes involving heat energy transfer.
- Understanding the specific heat capacity of different substances is crucial in various scientific and practical contexts.
- Effective measurement techniques such as calorimetry and advanced calculators allow for accurate specific heat calculations.
- Specific heat plays a crucial role across many sectors—engineering, cooking, and environmental science, promoting effective thermal management.
- Access to reference tables aids quick decision-making and analysis in various practical applications.
FAQ
1. What is the difference between specific heat capacity and heat capacity?
Heat capacity is the total amount of heat required to change the temperature of a given quantity of a substance, while specific heat capacity relates this to a unit mass of the substance. Therefore, specific heat is a more useful measure for comparing the thermal properties of different materials under the same conditions.
2. How can I calculate specific heat if I don’t have a calorimeter?
If a calorimeter is not available, you can still calculate specific heat using a known mass of a substance and its initial and final temperatures when subjected to a specific heat exchange with another known substance. This process can help understand the thermal interactions without specialized equipment.
3. Can specific heat values vary with temperature?
Yes, the specific heat values may vary with temperature. This means that a single substance may have different specific heat capacities at different temperatures. Therefore, it is important to reference the specific heat tables that provide values at standardized temperatures for accuracy.
4. Are there significant differences in specific heat between solids, liquids, and gases?
Yes, materials exhibit significantly different specific heats depending on their state; typically, gases have much lower specific heat compared to liquids and solids. This aspect can significantly affect heat management and energy transfer calculations in engineering and physical sciences.
5. How important is understanding specific heat in food science?
In food science, knowing the specific heat of various ingredients can help in both cooking efficiency and quality. It enables cooks and food scientists to ensure even cooking and avoid over-processing, leading to better textures and flavors in the final products.
By mastering the concepts of **specific heat**, you enrich your understanding of energy dynamics in physics and other scientific applications, paving the way for future innovations and developments in thermal technologies and methodologies.